Stereodivergent hydrodefluorination of gem-difluoroalkenes: selective synthesis of (Z)- and (E)-monofluoroalkenes†
Abstract
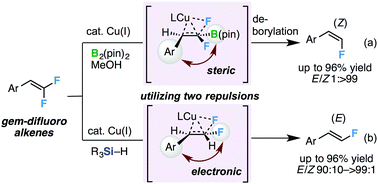
We have developed a novel approach for the stereodivergent hydrodefluorination of gem-difluoroalkenes using copper(I) catalysts to obtain stereodefined monofluoroalkenes. Both (Z)- and (E)-terminal monofluoroalkenes were obtained by the hydrodefluorination of gem-difluoroalkenes in the presence of copper(I) catalysts and diboron or hydrosilane, respectively, with high stereoselectivity. DFT calculations were conducted to elucidate the stereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...