Iodoarene-catalyzed oxidative transformations using molecular oxygen†
Abstract
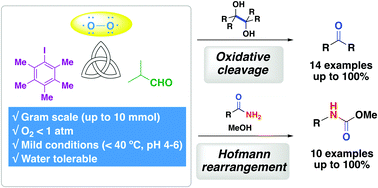
Molecular oxygen serves as a useful oxidant for the glycol scission of 1,2-diols and the Hofmann rearrangement of primary amides using pentamethyliodobenzene as a catalyst. The use of isobutyraldehyde and Lewis basic nitriles under O2 enabled the iodine(I)/(III) catalytic cycle, where in situ-generated peracid acts as a terminal oxidant.


 Please wait while we load your content...
Please wait while we load your content...