One-pot borylation/Suzuki–Miyaura sp2–sp3 cross-coupling†
Abstract
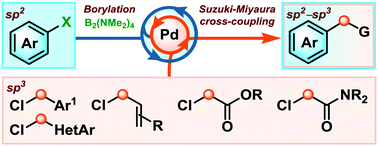
We describe the first one-pot borylation/Suzuki–Miyaura sp2–sp3 cross-coupling between readily available aryl (pseudo)halides and activated alkyl chlorides. This method streamlines the synthesis of diaryl methanes, α-aryl carbonyls and allyl aryl compounds, substructures that are commonly found in life changing drug molecules.


 Please wait while we load your content...
Please wait while we load your content...