In operando studies on the electrochemical oxidation of water mediated by molecular catalysts
Abstract
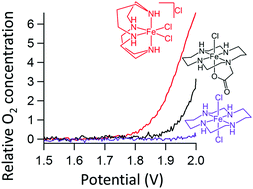
Homogeneous reactions in general are relatively easy to study with respect to heterogeneous systems since all catalytic sites are uniform and can be addressed simultaneously. The latter feature is fully out of the window in an electrochemical context, where only the few catalytic species that are sufficiently close to the electrode undergo redox reactions. Especially in the water oxidation reaction where harsh reaction conditions are employed, a clear picture of what is the active species, what products are formed, how one can steer this, and how it all depends on the exact reaction conditions is important to be able to fully unravel the key reaction paths. The combination of electrochemical experiments with on-line detection of the catalytic species and reaction products is a powerful approach to successfully address these questions. Recently, a significant progress has been made in on-line studies on molecular water oxidation catalysts during electrochemical experiments. These are reviewed here.


 Please wait while we load your content...
Please wait while we load your content...