Solvent-controlled selective synthesis of biphenols and quinones via oxidative coupling of phenols†
Abstract
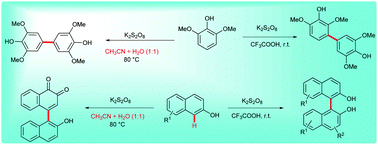
A regioselective synthesis of unsymmetrical and symmetrical biphenols and binaphthols via oxidative coupling of phenols or naphthols in the presence of K2S2O8 in CF3COOH under ambient conditions is described. Interestingly, the 1 : 1 ratio of H2O and CH3CN solvent mixtures at 80 °C instead of CF3COOH provided substituted unsymmetrical quinones. A gram-scale synthesis of biphenols and binaphthols was demonstrated.


 Please wait while we load your content...
Please wait while we load your content...