Catalyst-controlled site-selective asymmetric epoxidation of nerylamine and geranylamine derivatives†
Abstract
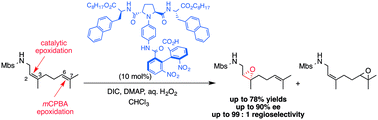
Novel catalysts for site- and enantioselective epoxidation of nerylamine and geranylamine derivatives have been developed. Although mCPBA oxidation took place selectively at the more electron-rich double bond to give the 6,7-epoxides, these catalysts provide the 2,3-epoxides in moderate to high enantioselectivity via the oxidation of the relatively electron-deficient double bond.
- This article is part of the themed collection: Site-selective molecular transformations


 Please wait while we load your content...
Please wait while we load your content...