Tri- and hexaferrocenyl-substituted subphthalocyanines in the quest for the optimum electron donor–acceptor distances†
Abstract
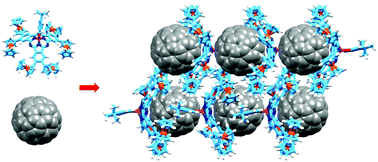
Three and six ferrocenyl subunits have been attached to the periphery of subphthalocyanines (SubPcs). Unlike axially coordinated ferrocenes, peripherally-bonded ferrocenes have an impact on the electronic features of SubPcs, which show a 44 to 70 nm red-shift of their Q-bands. The unusually deep and narrow ferrocenyl-SubPc is able to host C60, giving rise to atypical SubPc•C60 cocrystallates, through a combination of concave–convex and convex–convex π–π interactions.
- This article is part of the themed collection: Commemorating Michael Faraday (1791-1867)


 Please wait while we load your content...
Please wait while we load your content...