Total synthesis of 1α,25-dihydroxyvitamin D3 (calcitriol) through a Si-assisted allylic substitution†‡
Abstract
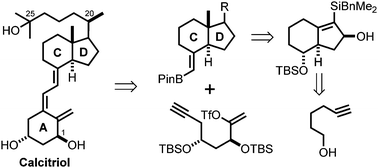
Herein, we describe a versatile and efficient total synthesis of 1α,25-dihydroxyvitamin D3 (calcitriol). The synthetic strategy relies on an unprecedented Si-assisted SN2′-syn displacement of carbamates by cuprates to set the challenging pivotal quaternary methyl group at the fused-ring junction of the CD-trans-hydrindane core. Other key transformations involve the catalytic asymmetric reduction of an α,β,γ,δ-unsaturated ester with CuH to generate the natural steroidal configuration at C20 and a Pauson–Khand cyclization to form the CD-ring skeleton. This strategy enables the syntheses of novel analogs for structure-function studies and drug development.


 Please wait while we load your content...
Please wait while we load your content...