Dissecting how modular polyketide synthase ketoreductases interact with acyl carrier protein-attached substrates†
Abstract
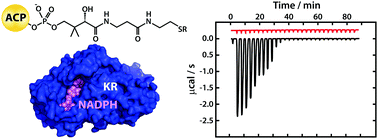
Interaction studies using fragments excised from the modular mycolactone polyketide synthase show that ketoreductase domains possess a generic binding site for acyl carrier protein domains and provide evidence that the pendant 5′-phosphopantetheine prosthetic group plays a key role in delivering acyl substrates to the active site in the correct orientation.


 Please wait while we load your content...
Please wait while we load your content...