Copper-catalyzed silylation reactions of propargyl epoxides: easy access to 2,3-allenols and stereodefined alkenes†
Abstract
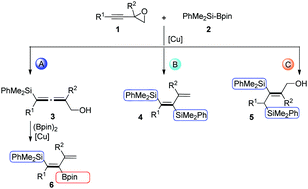
Efficient silylation reactions of propargyl epoxides catalyzed by copper catalysts have been developed. Under mild reaction conditions, tri- and tetra-substituted functionalized allenols and alkenes could be selectively obtained in moderate to high yields via tuning the bases and solvents used in the reactions. This work provides straightforward and efficient approaches to the synthesis of multifunctionalized 2,3-allenols and stereodefined alkenes from the same starting material of propargyl epoxides.


 Please wait while we load your content...
Please wait while we load your content...