Spectroscopic characterization of (diiodomethyl)zinc iodide: application to the stereoselective synthesis and functionalization of iodocyclopropanes†
Abstract
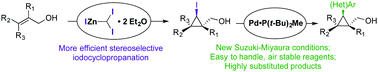
Herein is described an improved synthetic route to enantio- and diastereoenriched iodocyclopropylmethanols using (diiodomethyl)zinc iodide etherate as the active species. The products obtained by this methodology were successfully functionalized by Suzuki–Miyaura cross-coupling reactions. A Buchwald-type palladium precatalyst allowed access to highly substituted and stereo-enriched cyclopropanes without requiring a protecting group.


 Please wait while we load your content...
Please wait while we load your content...