Palladium-catalyzed aminocarbonylation of halo-substituted 7-azaindoles and other heteroarenes using chloroform as a carbon monoxide source†
Abstract
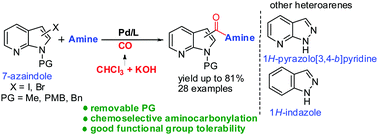
A palladium-catalyzed aminocarbonylation of halo-substituted 7-azaindoles utilizing CHCl3 as the carbonyl source has been developed for the straightforward incorporation of an amide functional group. The protocol was extended to other heteroarenes such as pyrazolopyridines and indazoles. The substrate scope of the reaction with respect to heteroarenes and the amine component is reported. This method offers an alternative avenue for aminocarbonylation of pharmaceutically important heterocycles.


 Please wait while we load your content...
Please wait while we load your content...