A mechanistic study of the non-oxidative decarboxylation catalyzed by the radical S-adenosyl-l-methionine enzyme BlsE involved in blasticidin S biosynthesis†
Abstract
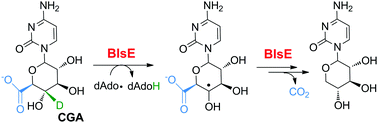
Decarboxylation is a fundamentally important reaction in biology and involves highly diverse mechanisms. Here we report a mechanistic study of the non-oxidative decarboxylation catalyzed by BlsE, a radical S-adenosyl-L-methionine (SAM) enzyme involved in blasticidin S biosynthesis. Through a series of biochemical analysis with isotopically labeled reagents, we show that the BlsE-catalyzed reaction is initiated by the 5′-deoxyadenosyl (dAdo) radical-mediated hydrogen abstraction from a sugar carbon of the substrate cytosylglucuronic acid (CGA), and does not involve a carboxyl radical as has been proposed for 4-hydroxyphenylacetate decarboxylase (HPAD). Our study reveals that BlsE represents a mechanistically new type of radical-based decarboxylase.


 Please wait while we load your content...
Please wait while we load your content...