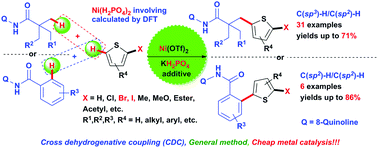
Nickel-catalysed direct alkylation of thiophenes via double C(sp3)–H/C(sp2)–H bond cleavage: the importance of KH2PO4†
Abstract
A Ni-catalyzed oxidative C–H/C–H cross-dehydrogenative coupling (CDC) reaction was developed for constructing various highly functionalized alkyl (aryl)-substituted thiophenes. This method employs thiophenes and aliphatic (aromatic) amides that contain an 8-aminoquinoline as a removable directing group in the presence of a silver oxidant. The approach enables the facile one-step synthesis of substituted thiophenes with high functional group compatibility via double C–H bond cleavage without affecting C–Br and C–I bonds. DFT calculations verify the importance of KH2PO4 as an additive for promoting C–H bond cleavage and support the involvement of a Ni(III) species in the reaction.


 Please wait while we load your content...
Please wait while we load your content...