Interconversion of molecular face-rotating polyhedra through turning inside out†
Abstract
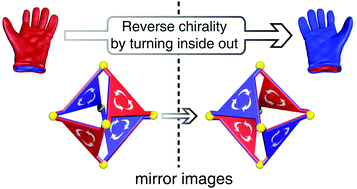
We report the post-synthesis interconversion of two enantiomeric organic cages through turning inside out. By scrutinizing the thermodynamics and kinetics, we are able to control the racemization rate by various reaction conditions and reveal that the turning-inside-out interconversion is realized through a partial disassembly pathway. The kinetics investigation also provides insight into the dynamic essence of imine chemistry using different solvents and catalysts.


 Please wait while we load your content...
Please wait while we load your content...