Highly efficient chemoenzymatic synthesis and facile purification of α-Gal pentasaccharyl ceramide Galα3nLc4βCer†
Abstract
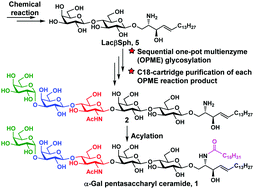
A highly efficient chemoenzymatic method for synthesizing glycosphingolipids using α-Gal pentasaccharyl ceramide as an example is reported here. Enzymatic extension of the chemically synthesized lactosyl sphingosine using efficient sequential one-pot multienzyme (OPME) reactions allowed glycosylation to be carried out in aqueous solutions. Facile C18 cartridge-based quick (<30 minutes) purification protocols were established using minimal amounts of green solvents (CH3CN and H2O). Simple acylation in the last step led to the formation of the target glycosyl ceramide in 4 steps with an overall yield of 57%.


 Please wait while we load your content...
Please wait while we load your content...