A naphthalene-fused dimer of an anti-aromatic expanded isophlorin†
Abstract
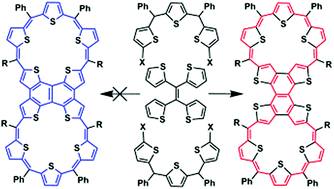
We report the first synthesis of a covalent expanded isophlorin dimer from two 24-π doubly S-confused sapphyrin-like pentathiaisophlorins. It exhibits marginal peripheral aromaticity rather than strong global diatropicity or paratropicity and weak intermacrocycle electronic communication. Quantum chemical methods discern that cross-conjugation is responsible for these unusual electronic features.


 Please wait while we load your content...
Please wait while we load your content...