Highly efficient synthesis of chiral aromatic ketones via Rh-catalyzed asymmetric hydrogenation of β,β-disubstituted enones†
Abstract
A succinct and efficient protocol was developed for the synthesis of chiral aromatic ketones via asymmetric hydrogenation of β,β-disubstituted enones with rhodium catalysts based on chiral bisphosphine thiourea ligands. A series of substrates (17 examples) was smoothly catalyzed to afford the corresponding chiral aromatic ketones in high conversions (>99%) with excellent enantioselectivities (up to 96% ee).
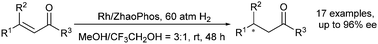


 Please wait while we load your content...
Please wait while we load your content...