An enantioselective cascade for simultaneous generation of five quaternary stereocenters from fully substituted enones†
Abstract
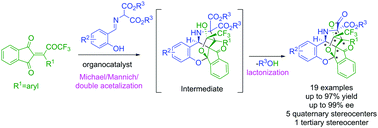
A highly enantioselective cascade reaction for the generation of five quaternary stereocenters in one-pot operation is reported for the first time in the history of organic synthesis. Cinchona-alkaloid derived hydrogen-bonding catalyst furnished structurally complex cascade products from simple substrates in excellent yields and stereoselectivities.


 Please wait while we load your content...
Please wait while we load your content...