Garlic-inspired trisulfide linkers for thiol-stimulated H2S release†
Abstract
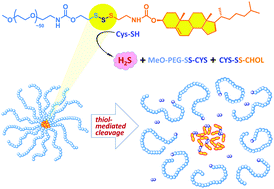
Garlic-derived polysulfides (e.g., diallyl trisulfide, DATS) act as potent donors of the cell-signalling mediator H2S when exposed to endogenous thiols. Inspired by this chemistry, we incorporated a trisulfide linkage into a conjugate comprising an mPEG tail and a cholesteryl head via thiol-mediated fragmentation chemistry. The synthesized conjugate releases H2S upon exposure to thiol even at intracellular levels.


 Please wait while we load your content...
Please wait while we load your content...