A precisely positioned chiral center in an i, i + 7 tether modulates the helicity of the backbone peptide†
Abstract
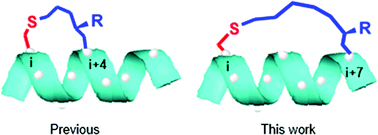
In some cases, helical peptides stabilized by an i, i + 7 tether exhibit better target binding and cellular functions compared to their i, i + 4 analogues. Herein, we carried out a systematic study of the effects of an in-tether chiral center on the i, i + 7 system. We screened the optimal cross linking mode, tether length, in-tether chiral center positions, and absolute configurations. From these studies, we determined that a chiral center of R absolute configuration at the γ-position to the C-terminal of a 10-membered tether could function to efficiently induce helicity of the backbone peptides. This is an important addition to the current i, i + 4 in-tether chiral center induced helicity strategy (CIH strategy), and could have broad biological applications.


 Please wait while we load your content...
Please wait while we load your content...