Metal-free formal carbon–halogen bond insertion: facile syntheses of 3-halo 3,3′-disubstituted oxindoles and spirooxindole-γ-butyrolactones†
Abstract
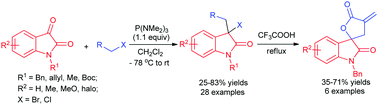
A novel P(NMe2)3-mediated formal carbon–halogen bond insertion of isatins into allylic and benzylic bromides/chlorides has been realized, leading to a facile synthesis of 3-halo 3,3′-disubstituted oxindoles. This reaction relies on the unique dual nucleophilic–electrophilic reactivity pattern of the Kukhtin–Ramirez adduct via a cascade SN2–SN2 process. It also represents a rare metal-free approach for carbon–halogen bond insertion. Upon treatment with trifluoroacetic acid, spirooxindole-γ-butyrolactones have been efficiently prepared from the corresponding insertion products.


 Please wait while we load your content...
Please wait while we load your content...