Facile access to 2,2-disubstituted indolin-3-ones via a cascade Fischer indolization/Claisen rearrangement reaction†
Abstract
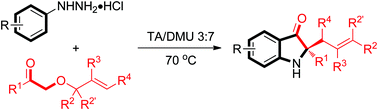
An efficient approach for the synthesis of 2,2-disubstituted indolin-3-ones is described. From readily accessible aryl hydrazines and allyloxyketones, 2,2-disubstituted indolin-3-ones could be obtained in good to excellent yields under mild reaction conditions via a cascade Fischer indolization/Claisen rearrangement process. This protocol provides a facile entry to 2,2-disubstituted indolin-3-ones, which have been applied in the construction of the benzofuroindoline framework related to Phalarine.


 Please wait while we load your content...
Please wait while we load your content...