Abstract
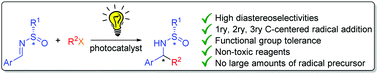
In this communication, a new photocatalytic strategy for the addition of alkyl-radical derivatives to N-sulfinimines with complete diastereoselectivity and moderate to good yields is presented. This is the first asymmetric photocatalytic addition to N-sulfinimines under visible light irradiation with smooth conditions and functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...