Desorption dynamics of CO2 from formate decomposition on Cu(111)†
Abstract
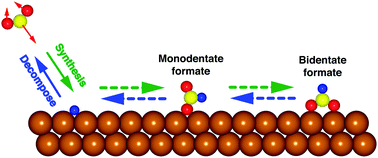
We performed ab initio molecular dynamics analysis of formate decomposition to CO2 and H on a Cu(111) surface using van der Waals density functionals. Our analysis shows that the desorbed CO2 has approximately twice larger bending vibrational energy than the translational, rotational, and stretching vibrational energies. Since formate synthesis, the reverse reaction of formate decomposition, has been suggested experimentally to occur via the Eley–Rideal mechanism, our results indicate that the formate synthesis can be enhanced if the bending vibrational mode of CO2 is excited rather than the translational and/or stretching vibrational modes. Detailed information on the energy distribution of desorbed CO2 as a formate decomposition product may provide new insights for improving the catalytic activity of formate synthesis.


 Please wait while we load your content...
Please wait while we load your content...