A visible-light-activated rhodium complex in enantioselective conjugate addition of α-amino radicals with Michael acceptors†
Abstract
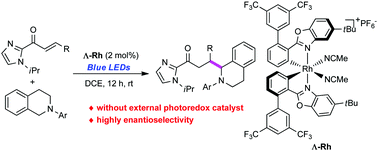
We report an efficient enantioselective conjugate addition of photogenerated α-amino radicals to Michael acceptors catalyzed by a newly prepared chiral-at-metal rhodium complex. This protocol shows that a single Rh(III) complex can serve not only as a Lewis acid but also as a photoredox catalyst to control the stereoselectivity during the bond formation.


 Please wait while we load your content...
Please wait while we load your content...