Facile access to unsymmetrically substituted tellurium–boron based heterocycles†
Abstract
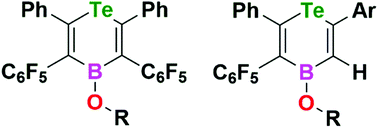
Reactions of Te–B heterocycles Te((Ph)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(C6F5))2B(C6F5) 1 with alcohols is shown to afford species of the form Te((Ph)C
C(C6F5))2B(C6F5) 1 with alcohols is shown to afford species of the form Te((Ph)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(C6F5))2BOR. The subsequent reaction with either 4-Br-C6H4CCH or 3-(C4H3S)CCH proceeds with the liberation of C6F5CCPh to give unsymmetrically substituted Te–B based heterocycles of the form Te((Ph)C
C(C6F5))2BOR. The subsequent reaction with either 4-Br-C6H4CCH or 3-(C4H3S)CCH proceeds with the liberation of C6F5CCPh to give unsymmetrically substituted Te–B based heterocycles of the form Te((Ph)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(C6F5))(HC
C(C6F5))(HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR)BOCH2Ph.
CR)BOCH2Ph.


 Please wait while we load your content...
Please wait while we load your content...