Enzymatic activation of cell-penetrating peptides in self-assembled nanostructures triggers fibre-to-micelle morphological transition†
Abstract
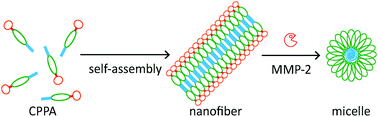
We report here a proof-of-concept design of a multi-domain cell-penetrating peptide amphiphile (CPPA) which can self-assemble into fibrous nanostructures and transform into spherical micelles upon enzymatic degradation by matrix metalloproteinase-2 (MMP-2) up-regulated in the tumour environment. Concomitant with this morphological transition, the cell-penetrating peptide (CPP), which was previously buried inside the CPPA fibers, could be presented on the surface of the CPPA micelles, enhancing their cell-penetrating ability. These multifunctional and enzyme-responsive CPP nanostructures hold potential as nanocarriers for tumour-targeted intracellular delivery of therapeutic and diagnostic agents.


 Please wait while we load your content...
Please wait while we load your content...