Gold-catalyzed oxidative hydroacylation reactions of α-iminoalkynes with aldehydes and O2†
Abstract
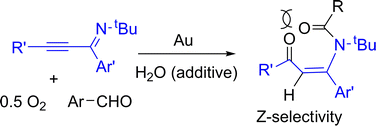
Gold-catalyzed aerobic oxidations of α-iminoalkynes with aryl aldehydes led to oxidative 1,3-hydroacyclation reactions, yielding high Z-selectivity. We performed 2H- and 18O-labeling experiments to confirm the role of water as an oxygen donor and O2 as the main oxidant. The Z-selectivity arises from an efficient conjugation between the enone and its aryl substituent. We postulate an initial [4+2]-annulation of α-iminoalkynes with aldehydes to form a six-membered oxonium species that is attacked by water, followed by the O2 oxidation of a key intermediate.


 Please wait while we load your content...
Please wait while we load your content...