With unprotected amino acids to tetrazolo peptidomimetics†
Abstract
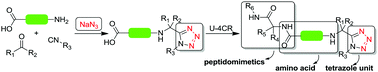
Here we describe the direct usage of C,N-unprotected amino acids in Ugi-tetrazole reactions to produce a novel class of acid-tetrazole compounds. Surprisingly, only the tetrazole Ugi product is found and not traces of other possible Ugi type reactions. Based on this reaction pathway we have designed the synthesis of novel tetrazole-peptidomimetics. A high level of structural diversity can be achieved using this isocyanide based multicomponent reaction (IMCR), providing a platform for the production of functionalized building blocks for novel bioactive molecules and nontraditional scaffolds which previously were not accessible.


 Please wait while we load your content...
Please wait while we load your content...