Nickel-catalyzed ring-opening of α-hydroxycyclobutenones with a remarkable ligand effect†
Abstract
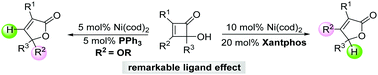
A Ni-catalyzed ring-opening of α-hydroxycyclobutenones is reported herein. A remarkable ligand effect was observed during transformations following the ring-opening. The employment of PPh3 leads to the formation of 2-furanones 2 through a migration of an alkoxyl group, and 2-furanones 3 were generated through a migration of hydrogen in the presence of Xantphos, affording a divergent approach to 2-furanones bearing multiple functional groups.


 Please wait while we load your content...
Please wait while we load your content...