Palladium-catalyzed intermolecular carbonylative cross-coupling of heteroaryl C(sp2)–H bonds with amines: an efficient strategy for oxidative aminocarbonylation of azoles†
Abstract
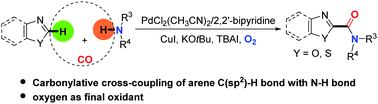
An efficient palladium-catalyzed oxidative aminocarbonylation of azoles has been developed. This system allows for intermolecular carbonylative cross-coupling of aromatic C(sp2)–H bonds with simple amines, which has often been asked for, but has not been realized so far. It provides a straightforward approach to a variety of azol-2-amides.


 Please wait while we load your content...
Please wait while we load your content...