Regioselective addition of C(sp3)–H bonds of alkyl pyridines to olefins catalysed by cationic zirconium complexes†
Abstract
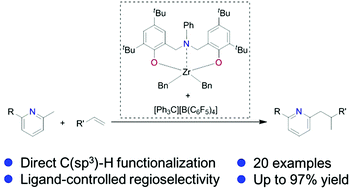
In situ generated cationic zirconium complexes stabilized by amine-bridged bis(phenolato) ligands have been developed to catalyse C(sp3)–H addition of alkyl pyridines to olefins, which are the first examples of group 4 metal based catalysts in this transformation. Ligand-controlled regioselectivity was observed, which was verified by DFT study.
- This article is part of the themed collection: 2017 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...