A biosynthetically inspired route to substituted furans using the Appel reaction: total synthesis of the furan fatty acid F5†
Abstract
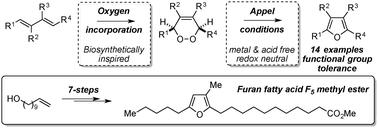
Appel reaction conditions have been harnessed to affect a mild biosynthetically inspired dehydration of endoperoxides to deliver multi-substituted electron rich furans. Unlike traditional dehydrative procedures, this method is metal and acid free, and can be achieved under redox neutral conditions. It is general for a range of aryl and alkyl substituted endoperoxides, and is functional group tolerant. Furthermore, this procedure has been used to deliver an effective total synthesis of the furan fatty acid (FFA) F5.


 Please wait while we load your content...
Please wait while we load your content...