Nickel-catalyzed hydrocarboxylation of ynamides with CO2 and H2O: observation of unexpected regioselectivity†
Abstract
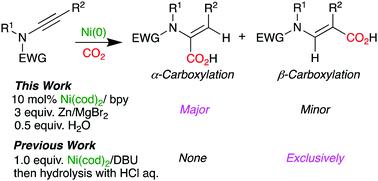
We describe the nickel-catalyzed hydrocarboxylation of ynamides with CO2 and H2O to afford a variety of α-amino-α,β-unsaturated esters with high regioselectivities. The selective α-carboxylation of ynamides with this catalytic protocol is unexpected in view of the electronic bias of ynamides and is in sharp contrast to our previous study in which a stoichiometric amount of Ni(0) was used to form a β-carboxylated product exclusively. We revealed that this unexpected C–C bond formation was induced by the combination of Zn and MgBr2.


 Please wait while we load your content...
Please wait while we load your content...