Cp*CoIII-catalyzed directed C–H trifluoromethylthiolation of 2-phenylpyridines and 6-arylpurines†
Abstract
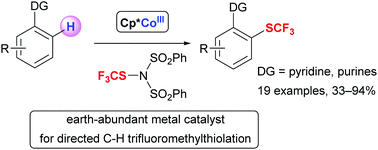
Cp*CoIII-catalyzed directed C–H trifluoromethylthiolation using N-trifluoromethylthiodibenzenesulfonimide as an electrophilic SCF3 source is described. 6-Arylpurines, an important structural motif in medicinal chemistry, and 2-phenylpyridines selectively afforded mono-trifluoromethylthiolated products in moderate to good yields using an inexpensive first-row transition metal catalyst.
- This article is part of the themed collection: Site-selective molecular transformations

 Please wait while we load your content...
Please wait while we load your content...