A cascade Claisen rearrangement/o-quinone methide formation/electrocyclization approach to 2H-chromenes†
Abstract
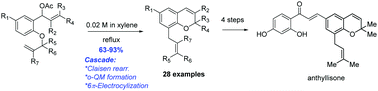
A new approach has been developed for the synthesis of 8-substituted 2H-chromenes, featuring a novel cascade aromatic Claisen rearrangement/o-quinone methide formation/6π-electrocyclization. This new method was demonstrated with 28 examples tolerating different substitutions at alkenes, allylic and aromatic ring and with total syntheses of three 2H-chromene natural products.


 Please wait while we load your content...
Please wait while we load your content...