Diastereoselective synthesis of novel heterocyclic scaffolds through tandem Petasis 3-component/intramolecular Diels–Alder and ROM–RCM reactions†
Abstract
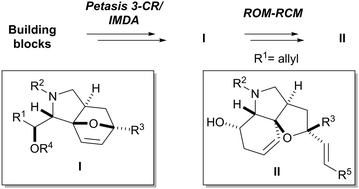
A high-yielding, stereoselective and extraordinarily complexity-generating Petasis 3-component/intramolecular Diels–Alder reaction has been developed. In combination with ROM–RCM, rapid access to complex sp3-rich heterocyclic scaffolds amenable to subsequent functionalization and library synthesis is provided.


 Please wait while we load your content...
Please wait while we load your content...