Palladium-catalyzed oxidative carbonylation of N-aryl enamino esters with CO and alcohols: synthesis of N-aryl aminomethylenemalonates†
Abstract
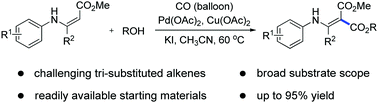
A novel palladium-catalyzed regioselective oxidative carbonylation of tri-substituted alkenes with CO and alcohols for the synthesis of α,β-unsaturated esters has been developed. Experimental studies and DFT calculations suggested that the reaction proceeded through alkoxylation of the palladium(II) catalyst, CO and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond migratory insertion, β-(N)H elimination and tautomerization cascade steps. The reaction tolerates a wide range of groups and produces valuable aminomethylenemalonates in high yields.
C double bond migratory insertion, β-(N)H elimination and tautomerization cascade steps. The reaction tolerates a wide range of groups and produces valuable aminomethylenemalonates in high yields.


 Please wait while we load your content...
Please wait while we load your content...