Rhodium-catalyzed C2 and C4 C–H activation/annulation of 3-(1H-indol-3-yl)-3-oxopropanenitriles with internal alkynes: a facile access to substituted and fused carbazoles†
Abstract
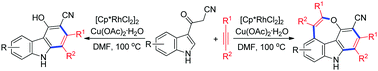
Rhodium-catalyzed oxidative annulation reactions of 3-(1H-indol-3-yl)-3-oxopropanenitriles with internal alkynes have been developed. A series of substituted carbazoles and 4H-oxepino[2,3,4,5-def]carbazoles, through a formal Rh(III)-catalyzed (4+2) cycloaddition with an alkyne and tandem (4+2) and (5+2) cycloaddition with two molecules of alkynes, were obtained. The reactions involved sequential cleavage of C(sp2)–H/C(sp3)–H bonds and annulation with an alkyne in the first step, and sequential cleavage of C(sp2)–H/O–H bonds and annulation with another alkyne in the second step. Some of the 4H-oxepino[2,3,4,5-def]carbazole products exhibit intense fluorescence in the solid state.


 Please wait while we load your content...
Please wait while we load your content...