Total synthesis of (±)-20S-hydroxy-1,2-dehydro-pseudoaspidospermidine via a C–H activation/transannular cyclization strategy†
Abstract
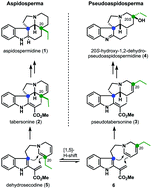
A total synthesis to the pseudoaspidospermidine family via a C–H activation/transannular cyclization strategy has been accomplished. The applicability of this approach is showcased in the concise synthesis (ten steps) of (±)-20S-hydroxy-1,2-dehydro-pseudoaspidospermidine (4) starting from literature known compound 11. Via a joint synthetic sequence we were also able to address the related iboga alkaloid (±)-isovelbanamine (7) in nine steps. Key features of this synthesis are a transannular cyclization to generate the pseudoaspidospermidine skeleton (C–H activation) and a Witkop photocyclization reaction providing a 9-membered lactam. It is also worth mentioning that the joint synthetic sequence can be carried out on a multigram scale.


 Please wait while we load your content...
Please wait while we load your content...