Copper-catalyzed remote (δ) C(sp3)–H bond amination: a practical strategy to construct pyrrolidine derivatives†
Abstract
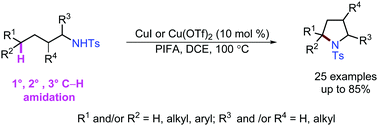
We report a copper-catalyzed remote C(sp3)–H bond amination reaction that converts acyclic amines to pyrrolidines. This reaction occurs selectively at the carbon δ to the amine functionality. Primary, secondary and tertiary C–H bonds are all suitable for the amination reactions in the presence of an inexpensive and commercially available copper catalyst.
- This article is part of the themed collection: Most downloaded articles of 2017: Catalysis


 Please wait while we load your content...
Please wait while we load your content...