Intermolecular cascade annulations of N-(arylsulfonyl)acrylamides with dual C(sp3)–H bonds: divergent access to indanes and pyrrolidin-2-ones†
Abstract
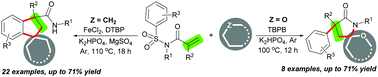
A new divergent intermolecular cascade annulation reaction of N-(arylsulfonyl)acrylamides with dual alkyl C(sp3)–H bonds for producing two types of five-membered rings, indanes and pyrrolidin-2-ones, is described. By using cycloalkanes and common alkanes as a one-carbon unit, an intermolecular [4+1] cascade carboannulation of N-(arylsulfonyl)acrylamides was achieved via a sequence of three C–H bond functionalization/aryl migration/desulfonylation that enables the formation of three C–C bonds and one N–H bond. When the one-carbon unit was changed to cycloalkyl ethers, the alternative intermolecular [4+1] cascade heteroannulation reaction occurred and allowed the construction of two C–C bonds and one C–N bond through dual C–H bond functionalization, aryl migration and desulfonylation cascades.


 Please wait while we load your content...
Please wait while we load your content...