Coordination strategy-induced selective C–H amination of 8-aminoquinolines†
Abstract
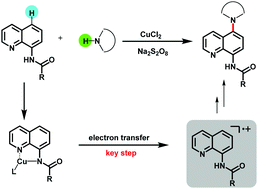
In this study, we broke through the directing function of the amide group. The coordination interaction between metal and directing-group enhanced the reactivity of the substrate. Using this strategy, we realized the selective amination of 8-aminoquinolines at the C5 position via employing azoles as the source of amine. Various kinds of 8-aminoquinolines and different substituted azoles were compatible to afford the corresponding C–N coupling products.


 Please wait while we load your content...
Please wait while we load your content...