Generating single metalloprotein crystals in well-defined redox states: electrochemical control combined with infrared imaging of a NiFe hydrogenase crystal†
Abstract
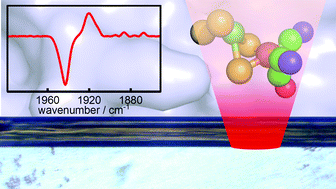
We describe an approach to generating and verifying well-defined redox states in metalloprotein single crystals by combining electrochemical control with synchrotron infrared microspectroscopic imaging. For NiFe hydrogenase 1 from Escherichia coli we demonstrate fully reversible and uniform electrochemical reduction from the oxidised inactive to the fully reduced state, and temporally resolve steps during this reduction.


 Please wait while we load your content...
Please wait while we load your content...