Rhodium-catalyzed synthesis of 1,2-dihydropyridine by a tandem reaction of 4-(1-acetoxyallyl)-1-sulfonyl-1,2,3-triazole†
Abstract
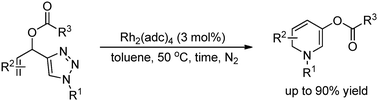
A tandem reaction of 4-(1-acetoxyallyl)-1-sulfonyl-1,2,3-triazole including formation of α-imino rhodium carbene, 1,2-migration of an acetoxy group and six electron electrocyclic ring closure was reported. The migration of the OAc group with excellent chemoselectivity was the crucial process, leading to the formation of 1,2-dihydropyridine specifically in up to 90% yield. Several transformations of the dihydropyridine product were also achieved illustrating the potential of the protocol in organic synthesis. Based on the observation of the intermediate, a plausible mechanism was proposed.


 Please wait while we load your content...
Please wait while we load your content...