CO2 reduction or HCO2− oxidation? Solvent-dependent thermochemistry of a nickel hydride complex†
Abstract
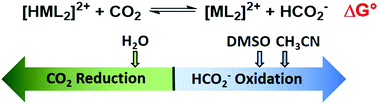
The hydricity (ΔGH−) of a newly synthesized nickel hydride was experimentally determined in acetonitrile (50.6 kcal mol−1), dimethyl sulfoxide (47.1 kcal mol−1), and water (22.8 kcal mol−1). The hydricity values indicate hydride transfer from [HNi(TMEPE)2][BF4] (TMEPE = 1,2-bis[di(methoxyethyl)phosphino]ethane) to CO2 is exergonic in water and endergonic in the organic solvents.
- This article is part of the themed collection: 2017 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...