N-Sulfonyl α-imino ester-derived chiral oxaziridines: catalytic asymmetric synthesis and application as a modular chiral organic oxidant†
Abstract
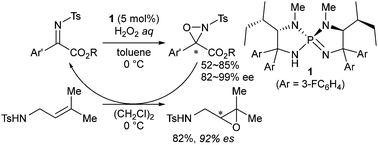
A novel class of chiral N-sulfonyl oxaziridines is introduced for use as structurally modifiable chiral oxidants. These oxaziridines are readily prepared from N-sulfonyl α-imino esters in a highly enantioenriched form by oxidation with hydrogen peroxide using L-isoleucine-derived triaminoiminophosphorane as a catalyst. The distinct advantage of their structural modularity is demonstrated through the identification of an optimal oxaziridine that exhibits high reactivity and enantiospecificity in the asymmetric oxidation of a silyl enol ether and N-sulfonyl allylic and homoallylic amines.


 Please wait while we load your content...
Please wait while we load your content...