Hemin-catalyzed sulfonium ylide formation and subsequently reactant-controlled chemoselective rearrangements†
Abstract
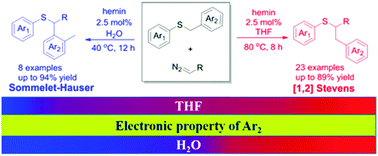
Hemin-catalyzed competing routes of [1,2]-Stevens and Sommelet–Hauser rearrangements of benzyl sulfonium ylides through an iron porphyrin carbenoid were established. These rearrangements can be controlled by the electronic property of the substituents on the benzyl group together with solvent effects. Electron-donating or weak electron-withdrawing groups lead to the [1,2]-Stevens rearrangement, whereas strong electron-withdrawing groups at the para-position favor the latter pathway under aqueous conditions.


 Please wait while we load your content...
Please wait while we load your content...