A general diastereoselective synthesis of highly functionalized ferrocenyl ambiphiles enabled on a large scale by electrochemical purification†
Abstract
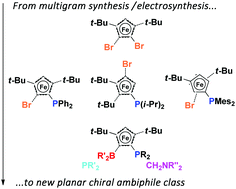
A general synthesis of highly functionalized ferrocenes, which include (P,B)- and (N,B)-ambiphiles, has been developed at a multigram scale. Diastereoselective stepwise modification of di-tert-butylated ferrocenes included the unprecedented separation of electroactive species. Bulky alkyl groups on ferrocenes ensure planar chirality of ambiphiles and enforce closer proximity of antagonist Lewis functions.


 Please wait while we load your content...
Please wait while we load your content...